Articular cartilage (AC), a biological tissue that protects and lubricates joints, plays a critical role during healthy locomotion. While much is known about this tissue's biochemistry and compressive mechanical properties, comparatively less attention has been given to its shear mechanical properties. This represents a critical knowledge gap because cartilage tissue experiences significant shear under normal loading conditions, and may indeed most frequently fail in such circumstances. In this study, we measured the depth-dependent complex shear modulus and energy dissipation rate for four anatomic sites in neonatal bovine knees. Our results demonstrated that there are significant differences in the mechanical properties among various regions, which may be crucial for allo/autograft transplant surgeries and tissue engineering applications.
The project and its major findings are summarized here, while details are available in the full publication.

Among other components, one of the major features to the microstructure of articular cartilage is a fibrous collagen network. This network has known variation as a function of position within the tissue. At the surface, the fibers can be aligned or randomly oriented depending on the anatomic location within the joint. Split-line testing is a standard technique that allows one to directly observe the local fiber orientation at the tissue surface. Shown here are digital photographs (left) and schematic overviews (right) of the split-line pattern found in the articular surface of neonatal bovine AC. The four regions studied are highlighted in red and referred to as the patellofemoral groove (PFG), the trochlea (TRO), the femoral condyles (FC), and the tibial plateau (TP).

To measure local shear mechanical properties, we use high-speed confocal strain mapping, a technique pioneered in the Cohen lab (see also Buckley+ 2010). Briefly, a tissue sample is clamped between two plates and a line is photobleached across it. This line acts as a fiducial marker and helps facilitate automated tracking of shear strain as a function of depth from the articular surface. Shear is then applied by one plate, while load is simultaneously measured at the opposing side. Deformations of the photobleached line are imaged at 20 Hz, and image tracking techniques are used to calculate the local dynamic modulus.
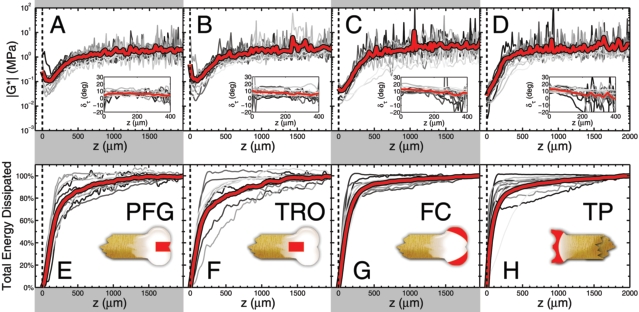
Measurements of the depth-dependent shear modulus |G*(z)| for the (A) PFG, (B) TRO, (C) FC, and (D) TP are plotted on a logarithmic axis as a function of depth z. The articular surface is at z = 0, gray lines are individual measurements (n = 13 samples for each anatomic region), and the red lines are averages. We find the first 500 mm of tissue is 10–100 times more compliant than the remainder of the tissue. Insets show the phase angle deltatau of the complex shear modulus. Measurements of the cumulative energy dissipated as a function of depth for the (E) PFG, (F) TRO, (G) FC, and (H) TP show that the tissue near the articular surface is primarily responsible for viscous losses. Insets schematically illustrate the region from which tissue was harvested. Collectively, these results highlight the significant depth-dependent variations that are exhibited by articular cartilage, and suggest a unique biomechanical role for the articular surface in absorbing energy during shear loading

From our depth-dependent measurements, we compared (A) Gmin, the minimum value of |G*(z)|, (B) Gbulk, the average value of |G*(z)| for z > 1,000 mm, (C) the percent of energy dissipated within the first 50 mm of the articular surface, and (D) the percent of energy dissipated within the first 500 mm from the articular surface. In each case, averages and standard deviations are shown for the four anatomic regions studied (n = 13 for each anatomic region). Here, * = p < 0.05, as determined from a balanced one-way ANOVA. These differences in the average values may be crucial for allo/autograft transplant surgeries or tissue engineered constructs: if the depth-dependent properties between an implant and native tissue are sufficiently mismatched, there may be considerable damage induced by irregular loading conditions and boundary effects.
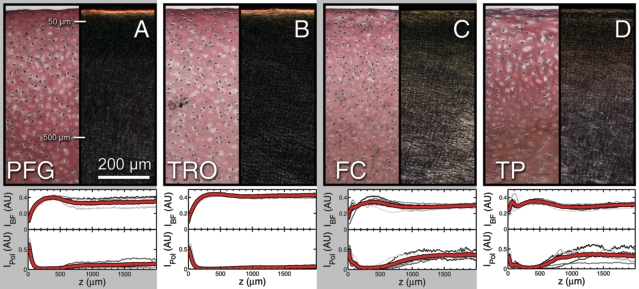
While depth-dependent variations were observed in AC mechanical properties, their microscopic origin remains unknown. To explore this, we stained cartilage sections with Picrosirius red, then imaged them with brightfield and polarized light under identical illumination conditions. The former conditions reveal collagen concentration such that regions of high collagen content are darker then regions of low collagen content. The latter conditions reveal collagen fiber orientation such that light regions have collagen fibers aligned parallel to the tissue surface (horizontal in the image), while dark regions indication collagen fibers oriented in the perpendicular direction. Representative images shown here for the (A) PFG, (B) TRO, (C) FC, and (D) TP illustrate the similarities and differences between the four regions. To facilitate comparison between anatomic regions, we averaged the depth-dependent pixel intensity for brightfield monochromatic (IBF) and polarized light (IPol), normalizing the data by the maximum possible intensity (arbitrary units). For each region, we plot these two semi-quantitative measures for individual images (gray) as well as the average (red; n = 6 for each anatomic region). All samples exhibit spatially localized depth-dependent variations of collagen density and fiber organization within the first 500 mm of tissue. Thus, while it is clear the depth-dependent collagen structure parallels the depth-dependent shear properties, the quantitative relationship governing structure and function remains unknown.
This work was accepted for publication in the Journal of Orthopaedic Research, 2012
